Slow Sand Filtration
by Mary Rust and Katie McArthur
 Introduction Introduction
The history of slow sand filtration in the United States has been one of
reluctant acceptance. Many European cities choose slow sand filtration as a
water treatment method because of its simplicity, reliability, and economy
(Collins). Slow sand filters in the United States are found primarily in smaller
communities with fewer than 10,000 people, 45% of which serve fewer than 1,000
people (Sims). This is primarily due to the associated low cost of slow sand
treatment facilities compared with alternative water treatment technologies
(Sims). The number of operating slow sand filters by state in the United States
as of 1991 can be seen in Figure 1.
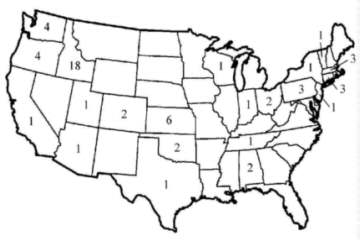
Figure 1. Number of
slow sand filters operating in each state as of 1991. (Sims)
 Physical Characteristics Physical Characteristics
Slow sand filtration is a water purification process in which water is passed
through a porous bed of filter medium. Slow sand filters are typically
characterized by certain design components: the supernatant (water above the
filter sand that provides hydraulic head for the process), filter sand varying
in depth, the underdrain medium (usually consisting of graded gravel), and a set
of control devices (Sims). In a mature sand bed, a thin upper sand layer called
a Schmutzedecke forms. The Schmutzedecke consists of biologically active
microorganisms that break down organic matter while suspended inorganic matter
is removed by straining (Van Duk). Slow sand filters are distinguished from
rapid sand filters by the biologically active sand medium (including the
Schmutzedecke), and slow detention times. Rapid sand filters utilize primarily a
physical removal process, are periodically backwashed for cleaning, and operate
with long detention times. Slow sand filters are cleaned by periodically
scraping the existing Schmutzedecke (Van Duk). Figure 2 is a schematic of a
common cross section of a slow sand filter.
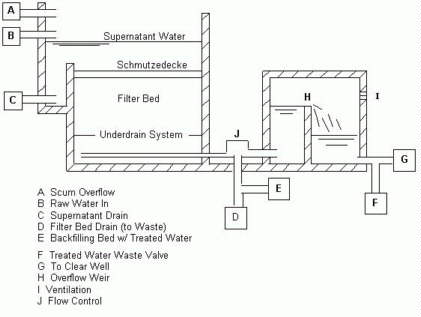
Figure 2. Typical
cross section of a slow sand filter.
Drawing by Mary Rust
The supernatant serves two distinct purposes. First, it provides a head of
water sufficient to pass the raw water through the filter bed. Second, the
supernatant creates a detention time of several hours for the treatment of the
raw water. The supernatant should not be considered as a reservoir for
sedimentation. If the raw water has a high content of suspended mater, then
pretreatment should be considered to prevent rapid clogging of the filter bed.
The supernatant depth is typically a meter (Van Duk).
The physical characteristics of a sand bed are important in maintaining the
slow sand filterís efficiency. The effective size is the size opening that will
pass ten percent by weight of the filter material (Haarhoff). Effective sizes in
the range of 0.15 mm to 0.35 mm are used (Van Duk). The uniformity coefficient
is the ratio of the size openings that pass sixty percent of filter material to
the size openings that pass ten percent of filter material, e.g. the effective
size (Haarhoff). Uniformity coefficients range between two and five; most
facilities maintain uniformity coefficients less than three (Haarhoff). The
filter medium itself should consist of inert and durable grains; sand should be
washed so that it is free of clays, loams, and organic matter. Depth of a filter
bed ranges between 1.0 and 1.4 meters (Van Duk). The clean filter medium from a
slow sand filter in a treatment plant near Paris, France can be seen in Figure
3.
 Photo by Dr. Robert Hoehn
Photo by Dr. Robert Hoehn
Figure 3. Clean slow sand filter without
supernatant water layer.
The underdrain system serves two purposes. It provides unobstructed passage
for the collection of treated water and it supports the bed of filter medium. It
is important that the underdrain system provide a uniform velocity over the
entire filter area (Van Duk). The underdrain gravel is placed so that the finest
gravel is directly underneath the sand and the coarsest gravel is surrounding
the underdrain pipes or covering the underdrain block (Pyper). This prevents the
filter sand grains from being carried into the treated water system. An example
of support media and underdrain system is shown in Figure 4.
 Photo by Dr.
Robert Hoehn Photo by Dr.
Robert Hoehn
Figure 4. Underdrain and support media.
 Biological and Physical Mechanisms Biological and Physical Mechanisms
Biological activity in the sand bed is not well understood. Scientists have a
vague idea of the processes involved, but specific interactions are still
unknown. Suggested biological removal mechanisms are predation, scavenging,
natural death and inactivation, and metabolic breakdown (Haarhoff). In the
Schmutzedecke, algae, plankton, diatoms, and bacteria break down organic matter
through biological activity. It has been hypothesized that as the raw water
passes through the bed, it constantly changes direction. Thus, the sand grains
develop a uniform sticky layer of organic material that absorbs to the particles
by various attachment mechanisms. The sticky layer around the sand grains is
biologically active (bacteria, protozoa, bacteriophages) and the organic
impurities are biologically converted to water, carbon dioxide and harmless
salts. According to a study by Collins, the bacterial concentrations in the
Schmutzedecke were a function of the elapsed time and potential for cell growth
rather than the filtration of free-living bacteria from the source water
(Collins). The biologically active section of the entire filter bed extends
0.4-0.5 m downward from the surface of the Schmutzedecke (Van Duk).
Physical processes are also inherent to slow sand filter mechanisms. As the
biological activity of the filter bed decreases, the physical processes of
adsorption and chemical oxidation are the primary mechanisms (Van Duk).
Adsorption accounts for removals that were traditionally thought to be purely
biological. For example, the removal of chlorinated organics and the
distribution of viruses are thought to follow adsorption isotherms (Haarhoff).
Furthermore, suspended inorganic matter may be removed by the physical process
of straining (Van Duk).
 Organic Carbon Removal Organic Carbon Removal
Adsorption and biodegradation are considered to be the primary natural
organic matter removal mechanisms (Collins). Literature cited by Collins
suggests that large hydrophobic-humic organic molecules are removed by
adsorption, and smaller organic molecules are removed by both adsorption and
biodegradation. The smaller hydrophilic material (carbohydrates, aldehydes, and
simple organic acids) are considered to be primarily removed by biodegradation.
A common oxidant for the treatment of water in the United States is chlorine;
the hydrophobic-humic organics (considered to be the more trihalomethane
reactive) were removed in greater than 80% of all comparisons of organic
parameters cited in the Collins study.
Another interesting aspect of the Collins paper was the fact that natural
organic matter and organic precursor material were a function of filter media
biomass: the greater the biomass, the greater the organic carbon removals. Three
US sand filters were compared; the West Hartford filters use a unique
Schmutzedecke cleaning procedure called the filter-harrowing cleaning technique
(discussed in more detail under Filter Scraping). This procedure allows for the
minimization of biomass removal from mature sand filters resulting in increased
removals of biodegradation and bioadsorption (Collins).
 Removal of Giardia and Cryptosporidia Removal of Giardia and Cryptosporidia
In the past decade the protozoan parasite Cryptosporidium parvum has
been recognized as a significant threat to public water supplies. The resistant
stage of Cryptosporidia is called an oocyst; this stage is relatively
untouched by a chlorination disinfection process. Slow sand filtration has been
looked at in numerous studies to determine the viability of this treatment
process for the removal of Cyrptosporidia. A study in England by Timms
found reductions of oocysts greater than 99.97%; the oocysts were found in the
filter media above 2.5 cm. Another study in British Columbia by Fogel
contradicts the aforementioned study. Fogel found removal efficiencies of 48%;
this figure is significantly different than the 100% removals Fogel cites from
previous literature. However, a point to note concerning the British Columbia
filters is that they were operating well out of the range of the recommended
design limits for the uniformity coefficient at 3.5 (Fogel). Furthermore,
temperature can adversely affect the performance of a slow sand filter; the
British Columbia filters were operating at extremely low temperatures of less
than 1įC (Fogel). Overall, the literature supports data that strongly suggests
slow sand filtration is a viable alternative for Cryptosporidia
removals.
Slow sand filters have also been proven highly efficient in removing
Giardia lamblia, a frequently identified pathogenic intestinal
protozoa. The same study by Fogel found that despite the uniformity coefficient
parameter and the low temperatures, Giardia removals were complete.
This data was further supported by literature cited by Fogel. Furthermore, fecal
and total coliform counts were below the detection limit, and the removal rates
were similar to Giardia removals (Fogel).
 Schmutzedecke Scraping Operations Schmutzedecke Scraping Operations
Scraping typically involves the removal of the Schmutzedecke and the
operation is site specific. Frequency of scraping depends on the available head,
the media grain-size distribution, the influent water quality, and the water
temperature (Letterman). Higher frequencies of scraping are associated with
increased water temperature, high solids concentrations in the influent, low
head, and small media pore size. A typical operation involves draining the
supernatant (usually by continuing filtration with no influent) to 20 cm below
the sand surface, skimming off one inch of the Schmutzedecke and associated
sand, and then filling the filter from the bottom of the bed using filtered
water to prevent air entrapment. The bed should be refilled until depth is
sufficient to continue normal operations (Letterman). A filter in the process of
having the Schmutzdecke scraped is shown in Figure 5.
 Photo by Dr. Robert
Hoehn Photo by Dr. Robert
Hoehn
Figure 5. Removal of Schmutzdecke.
The study
by Collins at West Hartford Treatment Plant suggests a unique way to scrape the
Schmutzedecke that minimizes the amount of biomass removed. The supernatant is
drained to a height roughly 30 cm above the bed. A rubber-tired tractor equipped
with a comb-tooth harrow is place on top of the filter to rake the sand;
simultaneously the filter drains are opened, causing a steady discharge of
overlying water. As the Schmutzedecke is loosened, the colloidal debris is
caught by the moving water and discharged at the filter surface drain. The
process is repeated as necessary by backflushing until the entire filter surface
has been harrowed (Collins).
 Summary comments by Dr.
Robert Hoehn on the use of slow sand filtration in the United States. Summary comments by Dr.
Robert Hoehn on the use of slow sand filtration in the United States.
If you are interested in the latest concerns about the operation of slow sand
filters in the United States you may want to take a look at the AWWA Slow Sand Filtration
Forum.
 References References
- Collins, Robin M., T. Taylor Eighmy, James M. Fenstermacher Jr., and
Stergios K. Spanos. "Removing Natural Organic Matter by Conventional Slow Sand
Filtration." Journal of the American Water Works Association 84.5
(1992): 80-90.
- Fogel, Doug, Judith Isaac-Renton, Ronald Guasparini, William Moorehead,
and Jerry Ongerth. "Removing Giardia and Cryptosporidium by Slow Sand
Filtration." Journal of the American Water Works Association 85.11
(1993): 77-84.
- Haarhoff, Johannes, and John L. Cleasby. "Biological and Physical
Mechanisms in Slow Sand Filtration." Slow Sand Filtration. New York:
American Society of Civil Engineers, 1991.
- Letterman, Raymond D. "Operation and Maintenance." Slow Sand
Filtration. New York: American Society of Civil Engineers, 1991.
- Pyper, Gordon R., and Gary S. Logsdon. "Slow Sand Filter Design." Slow
Sand Filtration. New York: American Society of Civil Engineers, 1991.
- Sims, Ronald C., and Lloyd A. Slezak. "Slow Sand Filtration: Present
Practice in the United States." Slow Sand Filtration. New York:
American Society of Civil Engineers, 1991.
- Timms, S., J.S. Slade, and C.R. Fricker. "Removal of Cryptosporidium by
Slow Sand Filtration." Water Science and Technology 31.5-6 (1994):
81-84.
- J.C. Van Duk and J.H.C.M. Oomer, "Slow Sand Filtration for Community Water
Supply in Developing Countries," Technical Paper No. 2, WHO
International Reference Centre for Community Water Supply, Voorburg (The
Hague), The Netherlands, 1978.
Send comments or suggestions to:
Student Authors: Katie McArthur, mailto:kmcarthu@vt.eduand Mary Rust, mrust@vt.edu
Faculty Advisor: Daniel
Gallagher, dang@vt.edu
Copyright © 1996
Daniel Gallagher
Last Modified: 02/24/1998 |